Does Hbr Have Dipole Dipole Forces
The strength of London forces are based on the total number of electrons and the area over which they are spread. Hydrogen bonds are the second strongest inter-molecular force providing about 1040 kJmol of energy.
What Intermolecular Forces Are Displayed By Hbr Quora
Electronegativity plays a role but there is an even bigger effect you are forgetting.

. Hence all of them posses dipole-dipole and London forces. HFHCl HBR and HI have permanent dipoles. London dispersion forces Van der Waals forces Permanent dipole-dipole forces.
In AsH3 the electronegativities are almost the same and the net dipole moment is about 022D. HBr is a polar molecule. The hydrogen bond is one of the strongest intermolecular force.
Since the molecule is polar dipole-dipole forces. Simple Answer - They Dont. The net dipole moment for HBr isnt very large 080D since H and Br have electronegativities which are fairly close together.
Unequal shared electrons lead to a polar molecule which exhibits dipole-dipole IMFs. So the strength of dipole-dipole forces in HBr will be between HCl and HI. Does HBr have London dispersion forces as its only intermolecular force.
The electronegative difference between H and Br is less than H and Cl but more than H and I. CCl4 molecular geometry is tetrahedral and its electron geometry is also tetrahedral. The bigger the element the less tightly it can hold onto the.
What is the weakest Imfa. So the strength of dipole-dipole forces in HCl will be between HF and HBr. Beside this why does h2s have dipole dipole forces.
Therefore it has dipole-dipole forces. There are also dispersion forces between HBr molecules. The electronegative difference between H and I is the lowest so its dipole-dipole forces are the weakest.
Therefore it has dipole-dipole forces. As you go down a group the shell number n increases meaning that the outer valence electrons the ones to make that bond with hydrogen are further and further away from the nucleus. Because HBr is polar and Br2 is nonpolar they will not dissolve in one another.
What is the molecular geometry of CCl4. CCl4 is nonpolar in nature but its bond is polar. No since its a polar compound its also considered to be polar.
What is the strongest type of intermolecular attractions between separate molecules of hydrofluoric acid. Since S is more electronegative than H each S H bond is polarized with the bond moments directed as shown. The bond angle of CCl4 is 1095º.
Does HBr have London dispersion forces as its only intermolecular force. Now you need to know about 3 major types of intermolecular forces. The strongest inter-molecular force is the ion to.
The major IMF in hydrogen fluoride HF is hydrogen bonding as hydrogen is bonded to fluorine. No since its a polar compound its also considered to be polar.
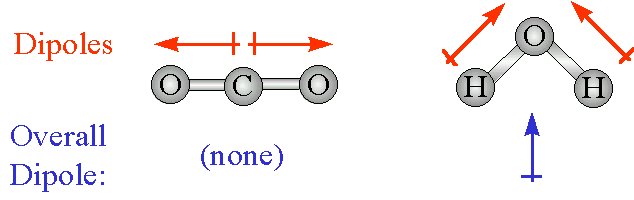
Which Of The Following Compounds Exhibits Only Dispersion And Dipole Dipole Intermolecular Interactions A Hbr B Co2 C H2o D N2 Socratic

Hbr Molecular Geometry Science Education And Tutorials

Best Overview Is Hbr Polar Or Nonpolar Science Education And Tutorials

No comments for "Does Hbr Have Dipole Dipole Forces"
Post a Comment